- +44 (0)330 124 7287
- Contact Us
- My Account
- Order Enquiry

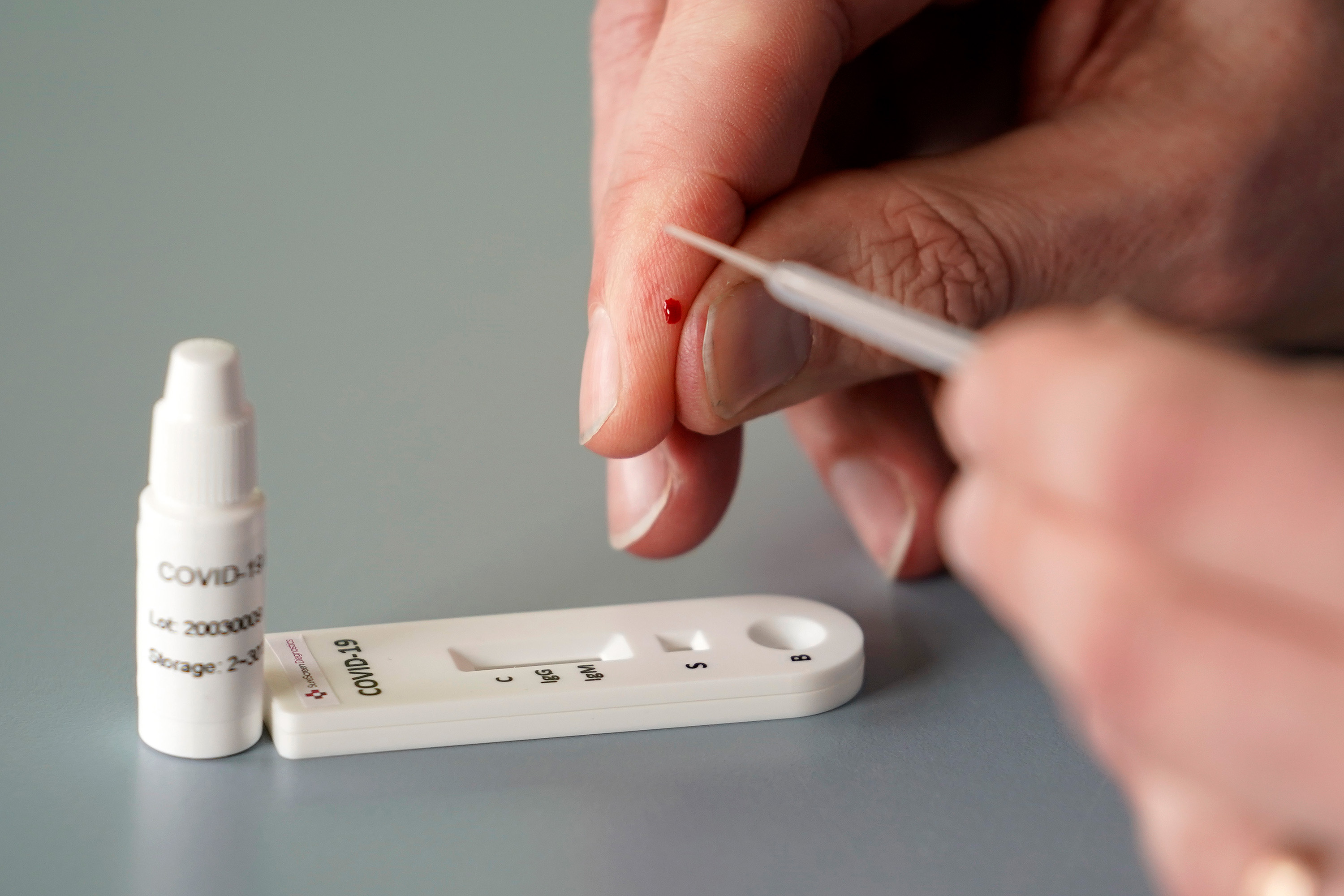
The SARS-CoV2 COVID-19 'ANTIBODY' Rapid Test Kits (LFT) are for testing to see if you HAVE HAD covid-19 or any of the related variants of the disease or if someone has received the covid vaccination.
This easy-to-administer 'Antibody' pin prick blood test will give the user a n extremely accurate positive or negative result within 15 to 30 minutes.
SPECIFICITY: 99.6% (IgG)
SENSITIVITY: 97.8% (IgM)
This test is CE marked for Professional use. The Clarity COVID-19 rapid test is manufactured by global eaders Roche and identifies the body's response to coronavirus after the onset of infection, and gives a qualitative yes/no result within 15 minutes.
The test cassette is easy to use, needing only a finger-prick sample to function, much like a blood glucose test.
As well as whole blood, the cassette can also be run with serum or plasma samples.
Product Includes: (1 Carton of 40 kits)
COVID-19 is an infectious disease that can affect your airways and lungs, caused by the most recently discovered coronavirus.
This infectious disease was unknown until the recent outbreak which originated in China in late 2019. At time of writing, cases of COVID-19 continue to rise daily across the globe.
The most common symptoms associated with COVID-19 are fever, tiredness and a dry cough, but symptoms can take up to 14 days to appear so it is difficult to know if someone has been infected ahead of these conditions appearing. In the meantime, infected people can spread the virus unknowingly.
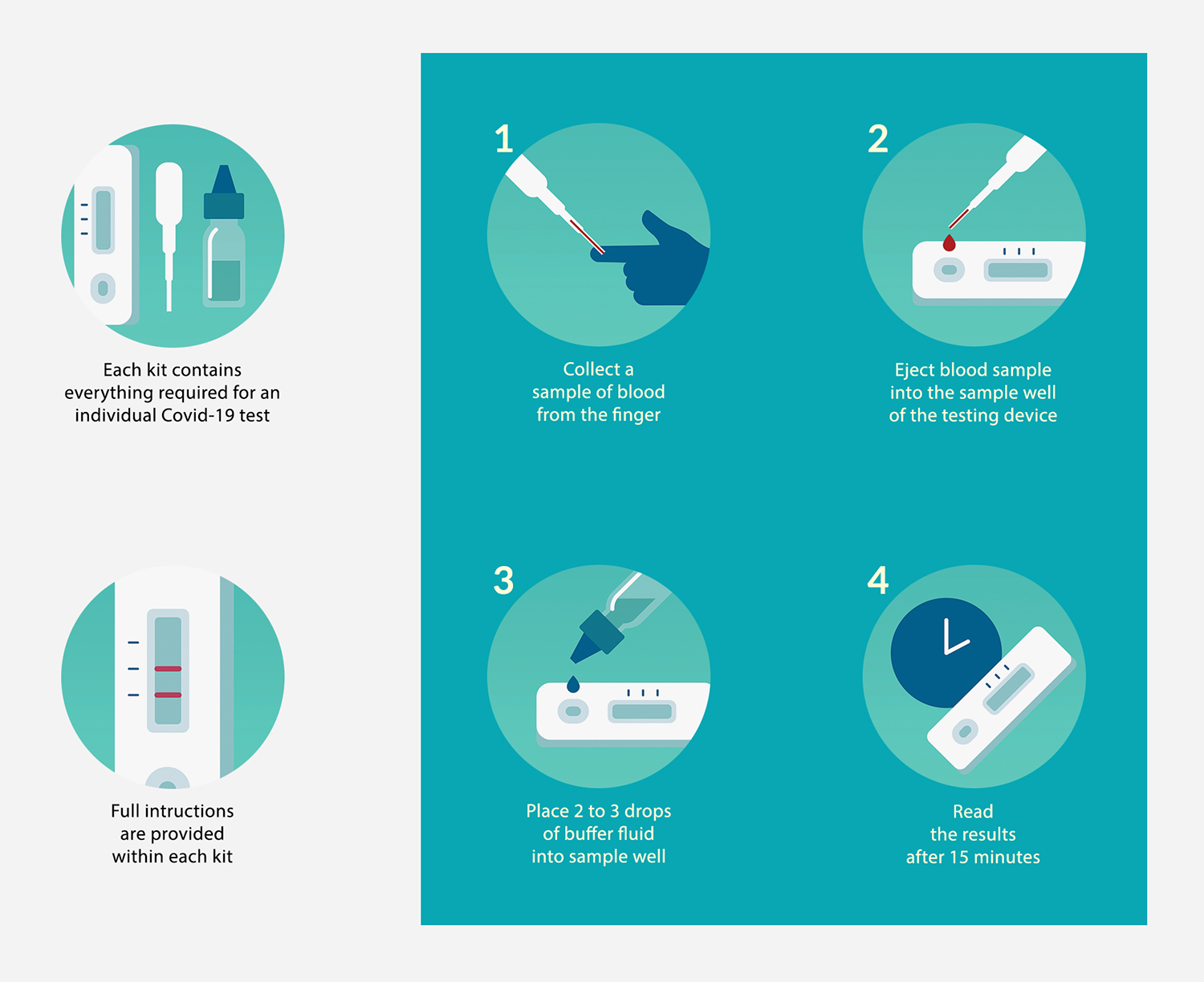
The test is a lateral flow immunoassay test and operates in a similar way to a pregnancy test. Embedded into the test strip are antibodies that bind to a COVID-19 specific biomarker, Immunoglobulin G (IgG), and another infection biomarker, Immunoglobulin M (IgM). When a sample (blood, serum or plasma) is added to one end of the test, it flows along the test strip and interacts with these antibodies. If the patient has contracted COVID-19, the biomarkers in their blood will bind to the antibodies on the test strip, leaving a visible test line. If the patient doesn't have COVID-19, no biomarkers should be present in their blood and no test line will be visible. There are separate test lines for IgG and IgM and only one needs to be visible for a positive result.
Immunoglobulins are antibodies themselves and are part of our immune system. When we get an infection, such as COVID-19, immunoglobulins are produced, which attach to the virus and activate the rest of the immune system to attack and clear the virus. IgM is the first immunoglobulin to be produced and is a general antibody that can bind to many different types of pathogen. The presence of IgM is an indicator of early infection. IgG is a more specialised antibody that specifically binds to the SARS-CoV-2 virus. The presence of IgG is an indicator of later stage infection (usually 7 days or longer after infection).
The IgG that the test detects is specific to COVID-19, so a positive result would indicate COVID-19 infection. Although the IgM is a more generalised antibody, its presence, combined with IgG and/or the common symptoms of COVID-19, would also indicate infection with the novel SARS-CoV-2 virus. The test can be used for primary and secondary diagnosis of COVID-19.
The test cassette will work with whole blood, plasma or serum. Capillary blood is the easiest to obtain via a finger pinprick, however, venous blood obtained via venepuncture is also suitable. The type of sample will not affect the sensitivity or accuracy of the test.
The buffer solution has two functions. It acts as the carrier for the sample, allowing it to laterally flow along the test strip, and it maintains a constant sample pH as it flows, preventing degradation of the biomarkers and the antibodies. We recommend that you only use the buffer solution provided with the test kits, as other ones may affect the accuracy of the test.
Yes, the test will be able to detect IgM/IgG in asymptomatic people, as they will have an immune response even though they don't display symptoms. The timeline of infection will be the same as someone displaying symptoms.
For the result, the window of accuracy is 10-15 minutes after you add the sample and buffer solution. If you forget to read the results after this time, the test may not be accurate and you should repeat it.
The shelf-life is 18 months from the date of manufacture. The product expiry is printed onto the packaging for reference. Do not use after the expiry date.
The recommended storage temperature is 2 - 30°C, however, we do not recommend you store them in the fridge, unless there is a risk of them overheating. Do not freeze the test cassettes or buffer solution. Tests should be performed at room temperature (15 - 30°C), so if you have stored tests in the fridge, you should allow them to reach room temperature before performing a test.
To date we have tested numerous common pharmaceuticals, none of which have interfered with the test. Also, Rheumatoid Factor does not interfere with the test, hence we currently see no cross-reactivity issues. We are looking at more drugs and will update customers accordingly.
We conducted a clinical study in Wuhan, China, where we compared the test cassette to conventional laboratory tests for COVID-19 diagnosis, which also detected the presence of IgG/IgM in 902 blood samples. We obtained the following results:
- Sensitivity > 91%
- Specificity > 99%
- Accuracy > 97%
This clinical study is evidence of the usability and effectiveness of the test.
There is some evidence that IgG/IgM remains in the blood after recovery to prevent reinfection. We have tested some patients and noticed that they still test positive for at least 33 days after first displaying symptoms. You won't be infectious after you recover, even if you test positive, but you should still be cautious as long term immunity has not yet been confirmed and reinfection may still occur.
Yes, there are no issues with testing babies and young children.
Yes, there is no harm to the mother or baby when performing a test.
No, the more people that are tested, the better it will be to understand the spread of the virus, which will result in better measures being taken to prevent its spread. However, as the test requires a blood sample, anyone with a blood-related health condition (such as haemophilia) should discuss this with a healthcare professional before performing a test.
Yes, the COVID-19 test is CE marked for professional use and is therefore a registered IVD device.
This is a relatively new test but is now being used successfully in many countries around the world.
The COVID-19 test is CE marked for professional use and is therefore suitable and ready for use by a healthcare professional. In addition to our clinical evaluations already completed on the product, we are currently working with a number of independent laboratories and Departments of Health across Europe to further validate the product and gain best practice approvals.
This screening test is designed to be used alongside a robust screening protocol with laboratory confirmations. We would recommend you work within your healthcare institution to ensure the correct protocols are being followed when using this product.
CALL: +44 (0)330 124 7287
CALL: +44 (0)330 124 7287